Chemical Properties of Amines
Chemical Properties of Amines: Overview
This topic covers concepts, such as, Chemical Properties of Amines, Basic Character of Amines, Basicity of Alkyl Amines versus Ammonia & Basicity of Aryl Amines versus Ammonia etc.
Important Questions on Chemical Properties of Amines
The increasing order of basic strengths in their aqueous solutions is
State which of the following statements are true:
(i) value for aniline is less than that for methylamine.
(ii) Methylamine in water reacts with ferric chloride to give a precipitate of ferric hydroxide.
(iii) Aniline does not undergo Friedel-Crafts reaction.
State which of the following statements are true:
(i) value for aniline is less than that for methylamine.
(ii) Methylamine in water reacts with ferric chloride to give a precipitate of ferric hydroxide.
(iii) Aniline does not undergo Friedel-Crafts reaction.
The order of basic strength in gas phase of the following compound would be:
The order of basic strength in gas phase of the following compound would be:
(i) Aniline does not undergo Friedel-Crafts Reaction.
(ii) Aliphatic amines are stronger bases than aromatic amines.
State if the above two statements are true or false:
Which one of the following is the strongest base in aqueous solution?
Amongst the following the most basic compound is –
Amongst the following the most basic compound is:
The correct order of increasing basic nature for the bases and is –
The correct order of increasing basic nature for the bases and is:
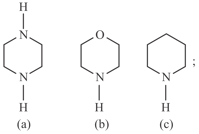
Compare basic strength of :
Which is the correct basic strength order of the following compounds?
i)
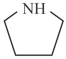
ii)
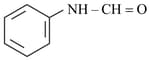
iii)
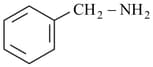
iv)
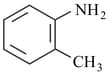
The decreasing order of basicity of following aniline derivatives is:
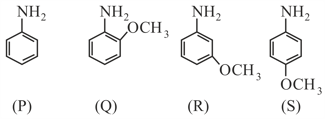
Which of the following compound has least Basic strength?
What is the increasing order of basic strength of the following compounds in aqueous solution?
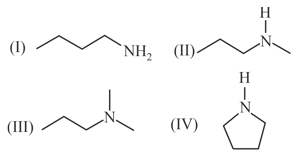
The correct increasing order of basic strength for the following compounds is _____.
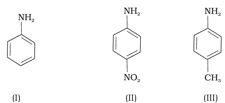
The most reactive amine towards dilute hydrochloric acid is _____.
Aniline is less basic than cyclohexylamine because of which of the following reasons?
Aniline is less basic than cyclohexylamine because of which of the following reasons?
